- Hernia Mesh
Hernia mesh recalls have become a growing concern for patients who rely on these medical devices for effective hernia repair. Designed to reinforce weakened tissue, hernia meshes are widely used in surgeries, yet numerous recalls have highlighted potential complications such as chronic pain, bowel obstruction, mesh migration, and life-threatening infections. With reports of defective products, lawsuits against manufacturers, and increased scrutiny from the Food and Drug Administration (FDA), it’s essential for patients and healthcare providers to stay informed.
In this blog, we explore the reasons behind hernia mesh recalls, the complications they cause, and the importance of working with an experienced attorney to navigate lawsuits and seek compensation.
Reasons for Hernia Mesh Recalls
Hernia mesh recalls occur for several reasons, primarily due to defective products and safety concerns. When a manufacturer discovers that their mesh devices do not meet safety standards or have design flaws, they may initiate a recall. This action is crucial to prevent further harm to patients who have undergone hernia repair surgery.
Common Causes for Hernia Mesh Recalls
Defective products can arise from various issues during the manufacturing process. For instance, some hernia mesh implants may contain materials that are not biocompatible, leading to adverse reactions in patients. Additionally, manufacturing errors can result in inconsistent mesh quality, which can affect its performance.
Safety concerns often stem from reports of complications associated with specific mesh products. If a significant number of patients experience issues such as chronic pain or recurrence of hernias, the FDA may step in to investigate. High failure rates can prompt manufacturers to voluntarily recall their products to protect patients.
Impact of High Failure Rates and Packaging Errors
High failure rates of hernia mesh products can have serious consequences for patients. Many individuals may require additional surgeries to address complications caused by faulty devices. This not only places a physical and emotional burden on patients but also leads to increased healthcare costs.
Packaging errors can also contribute to hernia mesh recalls. If the packaging fails to provide adequate instructions or warnings, healthcare providers may not use the device correctly. This can result in improper implantation and further complications for patients. Ensuring that packages contain clear and accurate information is essential for patient safety.
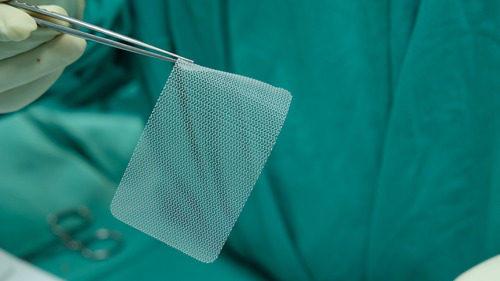
Types of Hernia Mesh Recalls
Hernia mesh products have been recalled for various reasons, often due to safety concerns and defects. Understanding the specific types of recalled devices is crucial for patients and healthcare professionals alike.
Atrium Medical C-QUR Mesh
The Atrium Medical C-QUR Mesh is a synthetic hernia repair product that faced recalls due to issues related to its packaging. Reports indicated that high humidity levels could compromise the mesh’s integrity, leading to potential complications. Patients using this product should be aware of the risks and consult with their healthcare providers.
Bard Composix Kugel Hernia Patch
The Bard Composix Kugel Hernia Patch has been linked to significant complications, including bowel perforation and chronic pain. This product was recalled after multiple reports of serious adverse events. If you have this patch implanted, it’s essential to discuss any concerns with your doctor.
Ethicon Proceed Surgical Mesh
Ethicon’s Proceed Surgical Mesh has also been under scrutiny for its performance. Recalls were issued due to concerns about the material’s safety and effectiveness. Patients have reported complications such as hernia recurrence and device migration. Those affected should seek medical advice promptly.
Physiomesh Flexible Composite Mesh
The Physiomesh Flexible Composite Mesh has been recalled due to high failure rates and serious complications. Many patients experienced issues such as infection and abdominal pain. If you have this mesh, monitoring your health and discussing any symptoms with a medical professional is vital.
Gentrix Surgical Matrix
Gentrix Surgical Matrix is another product that has faced recalls. Concerns regarding its long-term performance and safety have led to increased scrutiny. Patients should remain vigilant for any signs of complications and consult their healthcare provider if they experience any adverse effects.
Parietex Composite Parastomal Mesh
The Parietex Composite Parastomal Mesh has been recalled due to safety concerns that could lead to severe complications. Patients with this mesh should stay informed about hernia mesh recalls updates and ensure they receive appropriate medical care if complications arise.
Common Complications Associated with Recalled Hernia Mesh
Hernia mesh products, while designed to aid in the repair of hernias, can lead to a variety of complications. Patients who have received recalled devices may experience serious health issues that can significantly impact their quality of life.
Pain and Discomfort
One of the most prevalent complications associated with hernia mesh recalls is chronic pain. Many patients report ongoing discomfort at the surgical site. This pain can stem from the mesh itself, which may lead to inflammation or irritation in the surrounding tissue. In some cases, the pain can become debilitating, affecting daily activities and overall well-being.
Infection Risk
Infections are another significant concern for patients with recalled hernia mesh. The presence of foreign materials, like synthetic mesh, can increase the risk of infection. Symptoms may include swelling, redness, and fever. If an infection occurs, it may necessitate additional surgeries to remove the infected mesh and treat the underlying issue.
Hernia Recurrence
Unfortunately, some patients may find that their hernia recurs after surgery. This can be attributed to the failure of the mesh to properly support the abdominal wall or complications arising from the device itself. Recurrence can lead to further surgeries and extended recovery times, compounding the challenges faced by patients.
Bowel Obstruction and Migration
Severe complications can arise when the mesh migrates from its original placement. This migration can cause bowel obstruction, leading to symptoms such as severe abdominal pain, nausea, and vomiting. In extreme cases, this may require emergency surgery to relieve the obstruction and repair any damage to the intestines.
FDA Classification and Recall Process
Understanding the FDA classification and recall process is crucial for anyone affected by hernia mesh products. The FDA plays a vital role in ensuring that medical devices are safe and effective for public use. When issues arise, the agency can initiate recalls based on its findings.
Voluntary and FDA-Mandated Recalls
Recalls can be classified as either voluntary or mandated by the FDA. A voluntary recall occurs when a manufacturer identifies a defect or safety concern and decides to remove the product from the market. This action is often taken to protect patients and maintain trust.
On the other hand, an FDA-mandated recall is initiated by the agency when it determines that a product poses a significant risk to health. In such cases, the FDA will issue a formal recall notice, requiring the manufacturer to take immediate action. Both types of recalls aim to protect patients from defective products.
FDA Classifications
The FDA classifies recalls based on the severity of the risk associated with the device. The classifications include:
- Class 1 Recalls: This is the most serious type. It involves products that could cause serious health problems or even death. For instance, if a hernia mesh product is found to have a high failure rate leading to severe complications, it may be classified as a Class 1 recall.
- Class 2 Recalls: These involve products that may cause temporary or reversible health issues. While not as critical as Class 1 recalls, they still require attention. An example might be a hernia mesh product that has a defect but does not pose an immediate life-threatening risk.
The classification system helps patients understand the potential risks associated with their devices. It also guides healthcare providers in making informed decisions about patient care.
The Recall Process
Once a recall is issued, the manufacturer must notify affected healthcare providers and patients. This notification includes details about the recall, the reason for it, and instructions on what to do next. Patients should stay informed and follow any guidance provided by their healthcare providers regarding the recalled product.
Legal Actions and Lawsuits
Hernia mesh recalls have led to numerous legal actions against manufacturers. Patients who have experienced complications from these devices often seek justice through lawsuits. These legal claims can address issues stemming from defective products, including pain, infection, and other serious complications.
Overview of Lawsuits Against Hernia Mesh Manufacturers
Many patients have filed lawsuits against hernia mesh manufacturers like Ethicon, Bard, and Atrium Medical. These lawsuits typically argue that the manufacturers failed to ensure the safety and efficacy of their products. Common allegations include negligence, breach of warranty, and failure to warn about potential risks. As more patients come forward with similar experiences, the legal landscape continues to evolve.
Multidistrict Litigations
In response to the growing number of claims, several cases have been consolidated into multidistrict litigations (MDLs). MDLs streamline the legal process by combining similar cases in a single court. This approach can expedite proceedings and provide a more efficient resolution for all parties involved. It also allows for shared resources and evidence, which can strengthen the cases against manufacturers.
Potential Financial Compensation
Patients affected by recalled hernia mesh products may be eligible for financial compensation. This compensation can cover medical expenses, lost wages, and pain and suffering. If you have experienced complications from a recalled device, it is essential to consult with a legal professional who specializes in medical device litigation. They can help you navigate the complexities of filing a claim and determine the best course of action for your situation.
Seek Guidance For Your Hernia Mesh Lawsuit With Class Action Lawyer Coalition!
If you or a loved one have suffered complications from a recalled hernia mesh product, it’s crucial to take action to protect your rights. The physical, emotional, and financial toll of defective medical devices can be overwhelming, but you don’t have to face it alone. At Class Action Lawyer Coalition, our experienced team is dedicated to helping patients like you navigate the complexities of hernia mesh lawsuits.
Contact us at 855-938-0980 today for a free claim review!