A lawsuit can allow you to recover compensation if a defective medical device caused you injury. In many cases, however, you will have to work with an experienced mass tort attorney to get the compensation that you are entitled to.
Our law firm has educated legal professionals that can help with your transvaginal mesh litigation and always offer free consultations to our customers. To get more information, contact us today online or via 855-938-0980.
What is Transvaginal Mesh?
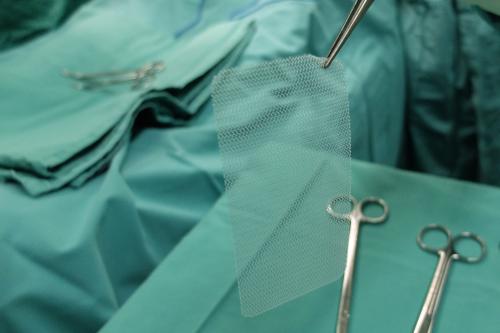
Transvaginal Mesh can also be referred to as bladder slings or pelvic mesh and is a mesh device that is implanted surgically used for treating stress urinary incontinence (SUI) and pelvic organ prolapse (POP) in girls.
POP happens when the muscles responsible for holding the pelvic organs in position become weakened thus leading to the bladder prolapsing or falling and pressing against the vaginal walls. SUI happens if an activity such as sneezing or coughing causes the leakage of urine out of the urethra.
The vaginal mesh is used for repairing the problems above by providing additional help to the damaged or weakened tissue. It’s typically made from biological substances or porous synthetic substances.
Surgical mesh has been in use since back in the 1950s, but it only began being used for treating abdominal repair of SUI in the 1990s.
The item, however, soon started causing difficulties and women started suffering debilitating side effects. A study conducted in 2017 indicated that around 40 percent of women suffer injuries due to the instruments used for implanting transvaginal mesh.
Artificial transvaginal mesh has advantages that occasionally pierce the surrounding tissues and can even puncture the gut, uterus, or bowel. The sling can also migrate and in the process wind up doing irreparable damage as it goes along. The mesh can also cause women to suffer nasty infections.
The synthetic mesh did not experience any serious security review by the FDA or other regulatory agencies prior to producers introduced it to the market. The most notable of those producers are Boston Scientific, American Medical Systems, C.R. Bard, Cook Medical, Coloplast, and Ethicon (a Johnson & Johnson subsidiary).
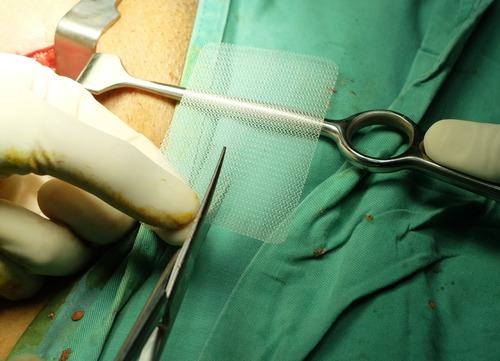
Transvaginal mesh surgery is done via the insertion of surgical mesh via the vagina as opposed to via the abdomen. While abdominal mesh surgery has its own issues, it’s still regarded as a much safer choice than transvaginal mesh surgery, which has been associated with severe, debilitating complications for thousands of girls.
The FDA warned of complications linked to transvaginal mesh implants back in 2008. The FDA kept expressing concerns and issuing warnings, with the most recent one being on January 4th, 2016 where transvaginal mesh operation for treating POP was reclassified from the FDA from moderate threat to high risk.
For the vast majority of those girls, the harm is already done.
What Will Be the Injuries Associated with Transvaginal Mesh?
The girls that girls allegedly have suffered in the mesh implant are painful and severe and may potentially have been prevented but only if these people had the right information.
The edges of the sling/mesh can easily cut through the skin following implantation thus cutting to nearby structures and causing infections and at times even puncturing the uterus, bowel, and bladder.
According to studies, the actual rate of harm is likely to grow considerably, which is hardly surprising since there are over 100,000 such procedures done each year. Reports made to the FDA imply there might be tens of thousands of injuries and hundreds of deaths.
Nonetheless, the accidents may take up to several years to grow after the initial operation. Tens of thousands of women have filed lawsuits alleging that pelvic mesh goods resulted in the injury.
Unfortunately, close to 30 percent of girls that underwent mesh operation for POP will need a second related surgery.
On account of the elevated risk of complications, vaginal mesh employed for treating POP was re-classified from the FDA because of a high-risk device. On the other hand, the classification does not apply to vaginal mesh employed for repairing SUI. Still, many surgeons have ceased using vaginal mesh completely as a result of associated risks.
Is It Possible to Remove Pelvic Mesh?
Safer synthetic mesh alternatives exist, such as biological mesh, which should be considered before deciding to use artificial materials.
Women that have these products in their bodies need to understand of the symptoms which indicate motion of the mesh so that they can tell when medical attention is needed. If you suffer from any symptoms or complications which you believe could be associated with a mesh implant’s positioning, let your physician known immediately. The most essential thing to do would be following the recommendation of a qualified physician.
Failed mesh implants nearly always require surgery to eliminate them. Nonetheless, this isn’t as simple as one might assume.
Once implanted, the mesh is incredibly hard to eliminate. Doctors are occasionally unable to eliminate all of the mesh after several surgeries since by that point it has become infused with all the surrounding tissue thus causing acute pain and life-threatening problems.
Who’s the Manufacturer of Vaginal Mesh?
The 4 manufacturers regarded as the top manufacturers of transvaginal products in the U.S.A. include:
- C.R. Bard
- Boston Scientific
- American Medical Systems (now a Unit of Endo Pharmaceuticals)
- Johnson & Johnson
Together, they confront tens of thousands of suits.
Has There Been a Transvaginal Mesh Recall?
Transvaginal mesh has not been recalled to the date, but on January 4, 2016, the transvaginal mesh employed in the treatment of POP was reclassified from the FDA as Class III high-risk devices. Class III is the maximum risk category the FDA has when it comes to medical devices.
Thousands of lawsuits have been filed by girls that obtained the transvaginal mesh against the producers. The recipients suffered chronic pain, serious and debilitating injuries, and often have experienced several surgeries with varying degrees of achievement to fix the harm or for the elimination of transvaginal mesh implant and protect against additional injury.
Doctors would previously utilize other choices when it came to repairing POP and SUI. The FDA now strongly recommends moving back to all those approaches whenever possible because the risks connected with transvaginal mesh are far greater than any possible benefits.
The FDA recommends that doctors will need to see it is possible to deal with POP without using mesh. Additionally, doctors will need to inform patients if the mesh is used. Patients must also be assertive when it comes to knowing why a Class III medical device is needed and what other choices are available.
Are Transvaginal Mesh Manufacturers Facing Lawsuits?
Yes. Tens of thousands of lawsuits have actually been registered by girls that obtained the transvaginal mesh contrary to manufacturers. The recipients endured chronic pain, serious and debilitating injuries, and in the majority of cases, have undergone several surgeries with varying levels of success to correct the damage or to the elimination of transvaginal mesh implant and prevent additional injury.
A lawsuit that was filed against Boston Scientific makes the allegation that the company actually used counterfeit resin from China in its vaginal mesh following a name-brand manufacturer ceased supplying resin for permanent implants due to security concerns. If these allegations are shown to be accurate, Boston Scientific is likely to face criminal charges.
How Can An Attorney Help A Transvaginal Mesh Claim?
In case you have suffered any discomfort or harm after obtaining a transvaginal mesh implant, you should think about joining tens of thousands of other women that have chosen to hold pharmaceutical manufacturers are most likely even others, in charge of their distress and harms that the transvaginal mesh apparatus have might have caused.
- Breach of Warranty
- Negligence
- Strict Liability
Based upon the jurisdiction where you file the lawsuit and the details of your case, you might also have other causes for legal actions. You may be entitled to recover compensation for:
- Loss of consortium
- Medical bills
- Lost wages
- Disability
- Pain and suffering
- Perhaps punitive damages (to discourage similar behavior and punish wrongdoers)
Federal courts haven’t awarded class-action standing to transvaginal mesh lawsuit, but lots of the lawsuits have been merged into multidistrict litigation (MDL).
After a federal judge rules on common legal issues concerning numerous transvaginal mesh suits, the litigation process can be focused when it comes to individual instances. This allows the jury to bypass the complex legal issues and instead hear and rule on questions of the fact that are different in your case, such as the way you were injured by the transvaginal mesh augmentation and what damage the injury did to your life.
Contact Our Lawyers for a Free Review of Your Case
You deserve to be paid for your pain and discomfort.
You may call us at 855-938-0980 today to schedule your no-risk, free case evaluation. We all know and understand the intricate universe of pharmaceutical legislation and can help you get the justice that you deserve. You can rely on us to help. Complete the online contact form or call us now!